https://www.npr.org/sections/health-shots/2022/01/25/ [login to see] /hospitals-use-a-lottery-to-allocate-scarce-covid-drugs-for-the-immunocompromised
Dr. Vivian Cheung takes steroids to manage a rare genetic disease. The drugs suppress her immune system, which puts her at high risk of getting very sick from COVID-19. It also means that her body didn't really make antibodies in response to two shots she got of the Moderna COVID-19 vaccine.
Cheung is a pediatrician and research scientist. Before the coronavirus pandemic, she flew weekly from her clinic at the National Institutes of Health in Maryland to her lab at the University of Michigan. Now she hasn't been to her lab in two years. "Except for work, I don't go out at all," she says. "I haven't been inside of a grocery store for over a year."
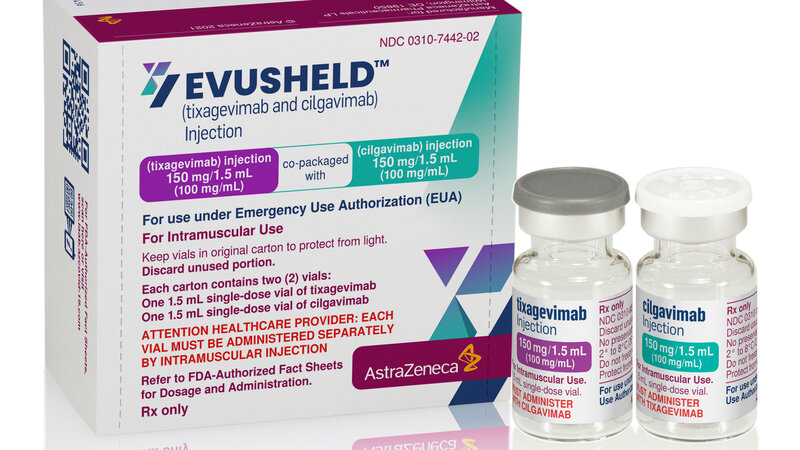
In December, the Food and Drug Administration authorized Evusheld, a monoclonal antibody combination from the drug company AstraZeneca that's designed to give patients like Cheung protection. For those who don't respond well to vaccines, Evusheld shots put COVID-fighting proteins directly into their bodies.
Analysis by AstraZeneca — completed last year — showed that the drug reduced the risk of getting COVID-19 by 77% and that the protection from a single two-shot treatment lasted for at least six months. Early data suggests it may work less well against the omicron variant of the coronavirus, but it is still expected to offer some protection.


 Medical
Medical Health
Health Drugs
Drugs Pharmaceuticals
Pharmaceuticals


