https://www.npr.org/sections/health-shots/2023/06/08/ [login to see] /fda-advisors-endorse-new-rsv-antibody-drug-for-babies
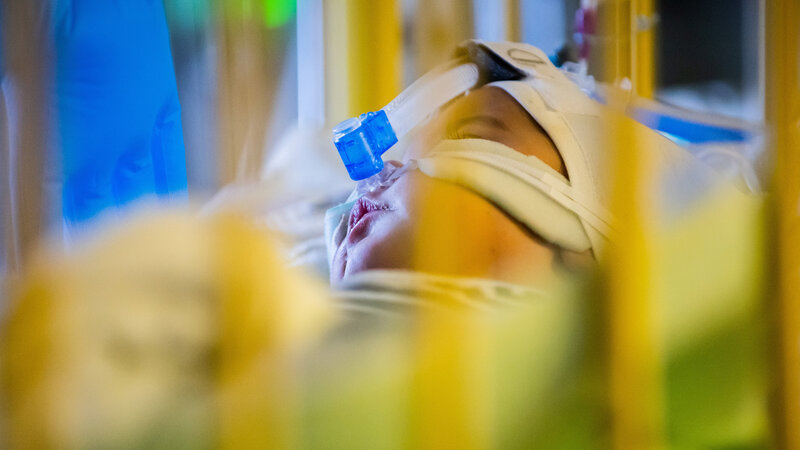
A panel of advisers to the Food and Drug Administration have recommended that the agency approve a new antibody drug to protect infants from serious lung illnesses caused by respiratory syncytial virus, also known as RSV.
On Thursday, the panel voted in favor of FDA approval for the injectable antibody medication – called nirsevimab – after hours of testimony from the drugmaker AstraZeneca, FDA scientists and the public. The question before the panel was whether the benefits of the treatment outweigh the risks.
The drug, if approved, would offer babies protection from the virus in their first RSV seasons with a single shot. It would be more affordable and more widely available than the single existing preventive drug – a monoclonal antibody shot called palivizumab – which requires monthly administration and is reserved for babies at high medical risk.
There was unanimous support on the 21-person committee for approving the drug's use in infants ahead of or during their first RSV season. And, in a separate vote, all but two members of the panel supported giving the drug to infants with medical risks through their second RSV season.


 Pharmaceuticals
Pharmaceuticals Medical
Medical Health
Health Children
Children


