https://www.npr.org/sections/health-shots/2023/03/29/ [login to see] /the-fda-may-soon-authorize-a-spring-round-of-covid-19-boosters-for-some-people
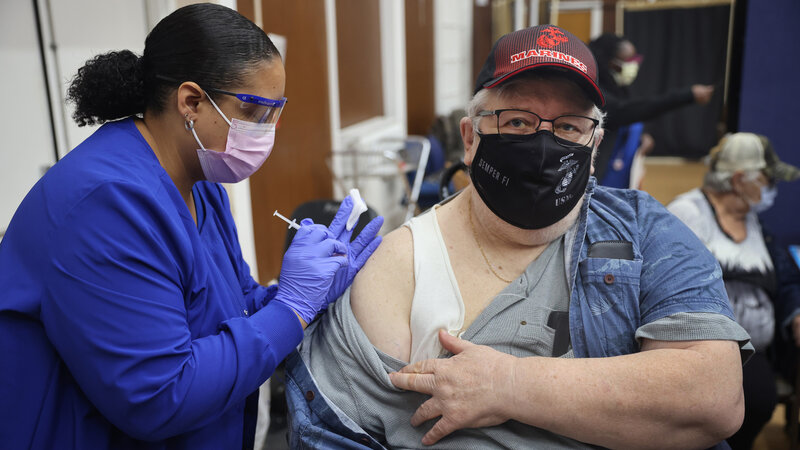
Federal health officials have greenlighted a second shot of the omicron booster for some older adults and those who are immunocompromised.
On Wednesday, advisors to the Centers for Disease Control and Prevention met and expressed support for allowing certain high risk groups to get a second shot of the updated COVID-19 vaccine — known as the "bivalent" vaccine because it targets both omicron and the original strain of the virus.
Following the meeting, the CDC issued a statement endorsing the new recommendations, which align with the Food and Drug Administration's recent decision to authorize a second shot. For now, the opportunity to a boost of the bivalent vaccine will be limited to those age 65 and older who got their first shot at least four months ago, and to those with weakened immune systems who got one of those shots at least two months ago.
Along with allowing a second shot for some adults, the CDC is also working to streamline its COVID-19 vaccine recommendations: Now most people are considered "up to date" if they've had one shot of the most current vaccine.


 Medical
Medical Health
Health Pharmaceuticals
Pharmaceuticals


