https://www.npr.org/sections/health-shots/2022/07/14/ [login to see] /over-the-counter-birth-control-pills
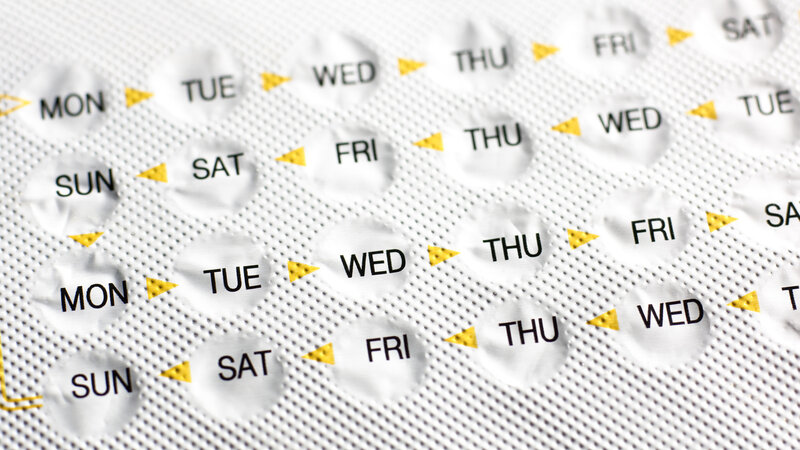
A pharmaceutical company based in Paris, HRA Pharma, is seeking approval from the U.S. Food and Drug Administration for an over-the-counter birth control pill. The pill includes progestin only, not estrogen, and is known as a mini pill. If approved, it would be the first oral contraceptive available in the U.S. without a prescription.
"This could be a really groundbreaking change in access," says Victoria Nichols of Free the Pill, a coalition of advocates, researchers and health care providers that has helped lay the groundwork and build support for regulatory approval of over-the-counter pill options in the United States.
The coalition's work began more than a decade ago, but the application for approval — submitted in the wake of the overturning of Roe v. Wade — comes at a time of renewed attention to the importance of contraception access. "I think there's absolutely greater urgency today to have better contraceptive access across the United States," says Cynthia Harper, a professor of obstetrics, gynecology and reproductive sciences at the University of California, San Francisco.


 Medical
Medical Health
Health Drugs
Drugs Pharmaceuticals
Pharmaceuticals Pregnancy
Pregnancy


